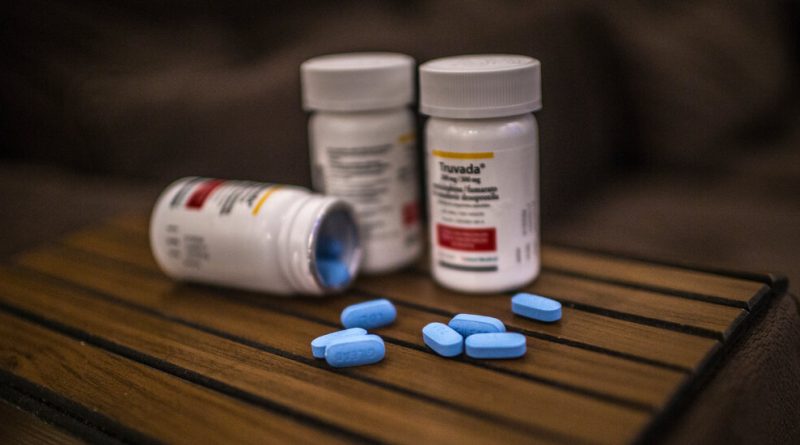
U.S. Loses Key Case on Rights to H.I.V.-Prevention Drugs
A federal jury in Delaware on Tuesday found that the federal government did not have an ownership claim to lucrative drugs to prevent H.I.V. that are sold by the pharmaceutical company Gilead Sciences.
The verdict in an unusual patent infringement case marked a defeat for the government and activists who have pushed it to more aggressively assert its financial and legal rights to medicines developed with the help of public funding. The Trump administration brought the lawsuit in 2019 in part because of concern over the high price Gilead was charging.
The legal dispute centered on who devised the idea of using a Gilead medication for people at high risk of contracting H.I.V., or the human immunodeficiency virus, which causes AIDS. The two versions of the drug — Truvada and the newer Descovy — have generated huge profits for Gilead.
Lawyers for the federal government had argued that Gilead had violated three government patents that protected the concept of using Truvada to prevent H.I.V. through what’s known as PrEP, or pre-exposure prophylaxis. The patents were granted to researchers at the Centers for Disease Control and Prevention for inventions stemming from experiments they conducted on monkeys in the mid-2000s.
But the jury found that Gilead had not violated any of those patents and that the company’s lawyers had proved that the government’s patented claims were invalid. The government had been seeking more than $1 billion in damages from Gilead.
Patent law experts said the government’s defeat in the case could embolden drug companies to refuse to enter into licensing agreements with the government to share in profits on medicines that grow out of contributions funded by taxpayers.
The United States already collects royalty payments for some inventions made by government scientists, but companies sometimes refuse to pay, claiming that the finished product was the result of private sector research and development.
In the case of Truvada, officials at the Department of Health and Human Services tried to get Gilead to license the rights to the C.D.C. patents, but the two sides never reached an agreement.
The company has said that it spent $1.1 billion on research and development related to Truvada.
Gilead’s general counsel, Deborah Telman, said in a statement that the jury’s verdict confirmed the company’s “longstanding belief” that it has always had the rights to the drugs for PrEP.
The six-person jury reached its verdict after a week of listening to dense scientific minutiae and testimony from leading H.I.V. experts. While drug companies commonly sue one another over patent disputes, the case appeared to be the first of its kind brought by the government, experts said.
Gilead was represented by Wilmer Hale, an elite corporate law firm. In his closing argument on Monday, Gilead’s lead lawyer, David Bassett, said the government had exaggerated the importance of an inexpensive “monkey study.”
“The government has acted like an adversary, a sharp-elbowed competitor that wants to claim for itself the right to use Gilead’s own drugs for PrEP,” he said.
Walter Brown, the lead lawyer for the federal government, told the jury the company had spent years “profiting handsomely” from the C.D.C.’s inventions, without paying back its fair share. Since 2017, which is the point at which the government said Gilead began infringing on the C.D.C. patents, the company has collected $10 billion in revenue from selling its drugs for PrEP in the United States.
In addition to the patented C.D.C. research, the government also spent about $143 million funding key clinical trials and other studies that paved the way for Truvada’s approval for use preventing H.I.V., according to a recent analysis.
H.I.V. activists said the public had paid multiple times for PrEP, first by contributing to its development and later by shelling out for the drug as Gilead repeatedly increased prices.
More than 30,000 people in the United States are newly diagnosed with H.I.V. each year. PrEP, taken daily as a pill, reduces the risk of infection by 99 percent and is seen as crucial to ending the H.I.V. epidemic.
Sahred From Source link Business