CPAP Maker Agrees to $479 Million Settlement Over Defects
[ad_1]
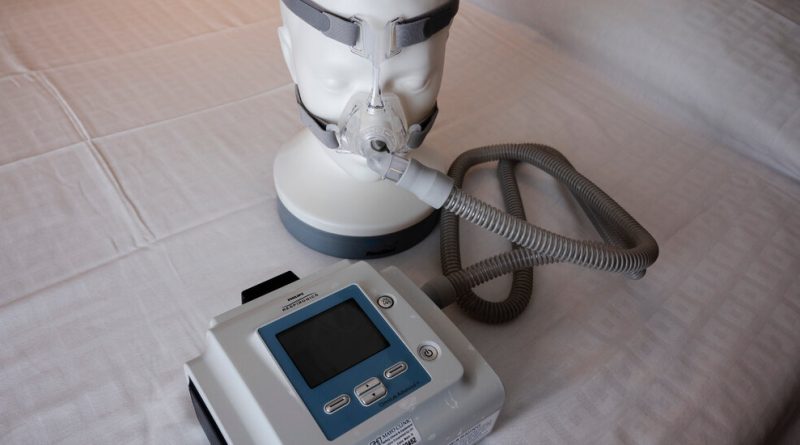
Philips Respironics has agreed to a $479 million partial settlement on claims over flaws in the company’s breathing machines that spewed gases and flecks of foam into the airways of consumers and that spawned recalls involving millions of the devices, lawyers for plaintiffs in the lawsuit announced on Thursday.
As one segment of continuing class-action lawsuits over the devices, the agreement covers only monetary reimbursements to users of the devices and vendors who might have financed replacements for consumers, according to the lawyers. The economic claims amount is uncapped, which will permit other device users to apply for compensation.
This tentative settlement, which is subject to federal court approval, does not address other significant claims in the plaintiffs’ cases involving personal injury or the cost of medical care related to use of the breathing machines. Philips did not admit wrongdoing or liability as part of the proposed deal.
The company has faced a multiyear setback, after beginning recalls in the United States of about five million of its breathing machines, which are intended for people with sleep apnea and other maladies. The lawsuits have claimed that flaking foam and gasses emitted from the machines were linked to health issues including respiratory illnesses, lung cancer and death. The foam was used in the machines to reduce noise and vibration.
In June 2021, the Food and Drug Administration announced a recall of Philips machines that also included BiPAP devices and ventilators made since 2009, warning that foam deterioration in the products could cause “serious injury” to users. Philips initially released a memo to doctors saying the foam breakdown posed risks of “toxic carcinogenic effects,” but the company has since released updates reporting a far lower level of concern.
“We are confident in these claims and we look forward to holding Philips accountable for the physical harms they caused patients,” the plaintiffs’ lawyers said in a statement.
Millions of people suffer from sleep apnea, a condition associated with interrupted breathing that carries a number of risks, including strokes, heart attacks and possible cognitive decline from decreased oxygen supply.
The spate of recalls in the last few years frustrated doctors and device users, who anguished over whether to continue using the machines and face potential health hazards, or forgo any treatment. Rival companies were hard-pressed to fill orders from those seeking replacements, leaving many consumers with no options.
The agreement announced on Thursday would provide compensation ranging from about $50 to $1,500 to each consumer, in addition to $100 for each device returned to Philips. The company said it replaced and delivered nearly 2.5 million devices for U.S. consumers and suppliers.
“Patient safety and quality are our top priorities, and we want patients to feel confident when using their Philips Respironics devices,” the company said in a statement.
The F.D.A. and some experts have criticized Philips for not notifying consumers when it first learned of potential flaws with some of its devices. Agency and court records show that concerns at Philips emerged in 2015. More than 105,000 injuries and 385 reports of deaths that were possibly related to the foam breakdown in Philips machines have been reported to the F.D.A.
The U.S. Department of Justice has been in touch with Philips about a possible consent decree to address problems related with the recall process, the company said in an earnings disclosure in July. Under a subpoena issued in April 2022 as part of another investigation into the events leading up to the recall, Philips continued to supply information, the July report said.