F.D.A. Experts Will Vote on Safety of a Cure for Sickle Cell Disease
[ad_1]
Why It Matters: A disease with a toll that is difficult to imagine.
An estimated 100,000 people in the United States have sickle cell disease, most of whom have African ancestry. Sickle cell shortens lives, injures organs and bones and causes episodes of searing pain that can repeatedly send patients to emergency rooms, or lead to lengthy hospital stays.
A report by the Institute for Clinical and Economic Review said that for people who don’t have sickle cell disease, “it is hard to understand the physical, emotional and mental toll.” People with the disease, the report added, “not only described intense fatigue, anxiety and depression, but at times extreme hopelessness.”
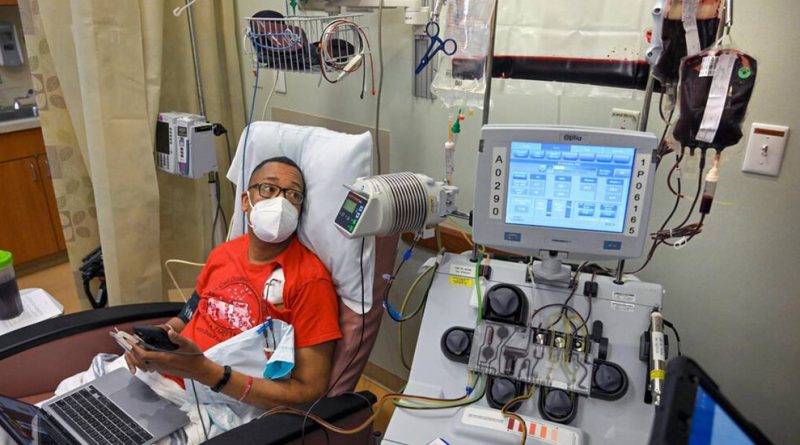
One patient, Mariah Jacqueline Scott, 32, who lives in Highland Park, N.J., has had two hip replacements, two shoulder replacements, a splenectomy, a gall bladder removal and a tonsillectomy because of the disease. She spent the year after her daughter was born in and out of the hospital being treated for extreme pain caused by blocked blood vessels. She had her second shoulder replacement after her shoulder collapsed while she was holding her baby.
The only cure has been a bone-marrow transplant, which requires finding a donor, undergoing intensive chemotherapy and taking immunosuppressive drugs. But gene editing offers an alternative. Vertex and CRISPR Therapeutics, the makers of the treatment being taken up by the F.D.A. committee on Tuesday, said that in clinical trials, symptoms of the disease went away after patients had the treatment. So far, the patients appear to be cured. The technique activates a gene that can make normally functioning blood cells.
Ms. Scott said she knew gene editing was arduous, but she was seriously considering undergoing the treatment when it became available.
Facts to Keep in Mind: Gene therapies bring their own challenges.
Vertex’s therapy starts when doctors remove stem cells from the blood and send them for treatment. Next comes intense chemotherapy to completely clear the bone marrow before the treated cells are injected. After that, patients must spend at least a month in a hospital while the treated cells repopulate the bone marrow.
Because each patient’s cells must be treated individually there are questions about how quickly companies can ramp up production.
“Manufacturing is very complicated,” said Dr. Stephan Grupp, chief of the cellular therapy and transplant section of Children’s Hospital of Philadelphia, who consults for Vertex.
Treatment will be extremely expensive, potentially in the millions of dollars per patient, and the companies will not say how many patients they expect to be able to treat at first.
Gene editing can also impose personal hardship on patients and their families. A hospital with the expertise to administer the treatment and care for patients may be far from home. And patients must stay there for a long period of time.
What’s next: More F.D.A. decisions and more drugs.
If the advisory committee recommends the Vertex treatment, the F.D.A. will decide whether to approve it on Dec. 8.
On Dec. 20, the F.D.A. will decide on another application for sickle cell gene therapy made by Bluebird Bio. Two other companies and an academic center, Boston Children’s Hospital, are testing their own sickle cell gene therapies.
While these therapies could reduce the suffering of sickle cell patients in the United States and other wealthy countries, there is an even greater need for them in some developing countries like Nigeria. However, they will be difficult to export to developing countries because the treatments are extremely expensive and they can only be administered at hospitals where doctors have expertise in a number of advanced techniques.
One company, Beam, is testing a way to provide gene editing that requires nothing more than a single infusion in a doctor’s office. Vertex has what it calls an “aspirational” method that would deliver gene editing in a pill.